Which of the following statements are true?
Discipline: Chemistry
Type of Paper: Question-Answer
Academic Level: Undergrad. (yrs 3-4)
Paper Format: APA
Pages: 1
Words: 275
Question
:
Which
of the following statements are true? If a statement is true only under
special circumstances, label it as false. Select all that apply.
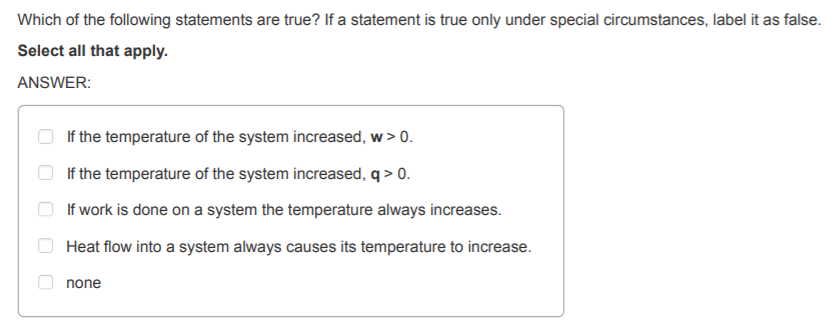
ANSWER: If the temperature of the system increased, w>O If the
temperature of the system increased, q>0 If work is done on a system
the temperature always increases. Heat flow into a system always causes
its temperature to increase. none
Expert Answer
Answer
None
Explanation
According to first law of thermodynamics
∆U = q + w
where
∆U = internal energy of system
q = heat released or absorbed by the system
w = work done by or done on system
From the first law of thermodynamics we know that ∆U is depends both on q and w but not resticted only to either q or w.